Mix well and store in a stoppered amber colored bottle. 1 Add approximately 030 grams of starch to 10 mL of water in a beaker.

Pdf A Comparison Of Iodine Values Of Some Co
Different chemicals and equipment were used in the experiment to determine the iodine value of the oil sample.
. The iodine value is the mass of iodine in grams that is consumed by 100 grams of a chemical substance. Pour a 2 cm depth of cyclohexane into one test tube. The iodine monochloride halogenates the double bonds in the fat and the residual ICl is reduced to free.
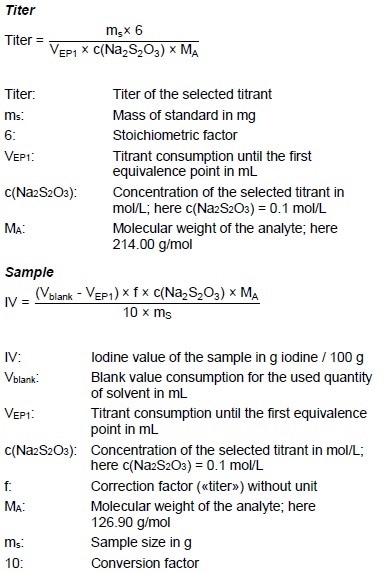
BDetermination of iodine value of supplied oils olive oil Volume of 01 N Na2S2O3 solution used for blank X ml Volume of 01 N Na2S2O3 solution used for oil Y ml Iodine number x. Normality of sodium thiosulphate Na 2 S 2 0 3 01. Decrease in iodine value shows decrease in the number of double bonds and it indicates oxidation of the oil.
X BC where X mL of bromine solution required to double the halogen. A rapid and practical method for determining the iodine value IV of edible oils or fats with an FTIR spectrometer combined with a quartz cuvette was reported. Dilute 50 ml of the stock to 1000 ml using Glacial acetic acid.
Blank solution and oil solution were prepared and stored in the dark. The quantity of substance used in the determination should be such that at. Equivalent Weight of Iodine 127.
Select two small crystals of iodine of the same size do NOT touch the crystals iodine stains skin and clothes and put one into each of two test tubes. The protocol for iodine value determination usually comprises a titration procedure such as the Wijs method 61. This is the stock solution.
Iodine value or number is the number of grams of iodine. It is expressed in gms the amount of iodine which is taken up by 100 gms of the fat wax. The determination is conducted by dissolving a weighed sample in a non- polar solvent such as.
The determination of iodine value is a particular example of iodometryA solution of iodine I 2 is yellowbrown in color. Calculate the value of bromine solution to double halogen content of the remaining 800mL of the above iodine solution as follows. Iodine numbers are often used to determine the amount.
In this procedure iodine chloride is used for double-bond saturation analysis and the. There are different methods for checking the unsaturation level in fatty acids one among them is by determining the iodine value of fats. The iodine number may be calculated from the volume of the oil taken specific gravity of the oil and the volume of Hubls iodine required to impart its colour to the solution of oil.
The sample was diluted with carbon. The exact amounts are not critical. Mix well and store in a well stoppered clean and amber.
Iodine monochloride ICl in acetic acid that reduces absorption time to approximately half an hour. Calculate the iodine number using the equation below. Determination of the iodine value 2341 The results of a statistical evaluation of the iodine values obtained in the 1st collaborative study in which 11 laboratories from 9 countries participated are.
In the titration of sample and blank if a starch solution was added to the flask with a lighter. Determination of the Iodine number according to Wijs. Dilution of gram iodine.
The iodine number determines the number of double bonds of the fatty acids present in a fat or oil. Add a magnetic stirbar and place on a magnetic stirplate. Volume of Sodium thiosulphate used Blank- Test ml.
The iodine value of a substance is the weight of halogens expressed as iodine absorbed by 100 parts by weight of the substance. The iodine value also known as the iodine number is a measure of the degree of unsaturation of fat wax or oil. S volume in ml of standard sodium thiosulphate solution.
Iodine value 1269 B S NW Where B volume in ml of standard sodium thiosulphate solution required for the blank. When this is added to a solution to be tested however any chemical group. It is a measure for the unsaturated.
Hanus Method In this experiment iodine value of sun floweraoil was determined with Hanusamethod.
Pdf Determination Of Iodine Value Of Palm Oil By Differential Scanning Calorimetry

Pdf Fast Spectrophotometric Determination Of Iodine Value In Biodiesel And Vegetable Oils

Solved Questions 1 Why Is Ki Added To The Reaction Chegg Com
The Determination Of The Iodine Number Of

Lab Report Example On Titrations Lab 3 The Iodine Number Of Fat Dom 99190758 Aim To Discover The Studocu
Pdf Determination Of Iodine Value Of Palm Based On Triglyceride Composition

Pdf Iodine Value In Partially Hydrogenated Castor Oil Ricinus Oil As Determined By Aocs Official Method Cd 1 25 Wijs Method

Determination Of Iodine Value A Complete Procedure Aoac 920 159 Youtube

Analysis Of Edible Oils And Fats
Solved A Fat Has An Iodine Value Of 250 What Can You Conclude About The Course Hero

Pdf Determination Of Iodine Value In Triisocetyl Citrate Citmol 316 By United Satates Pharmacopeia Hanus Method
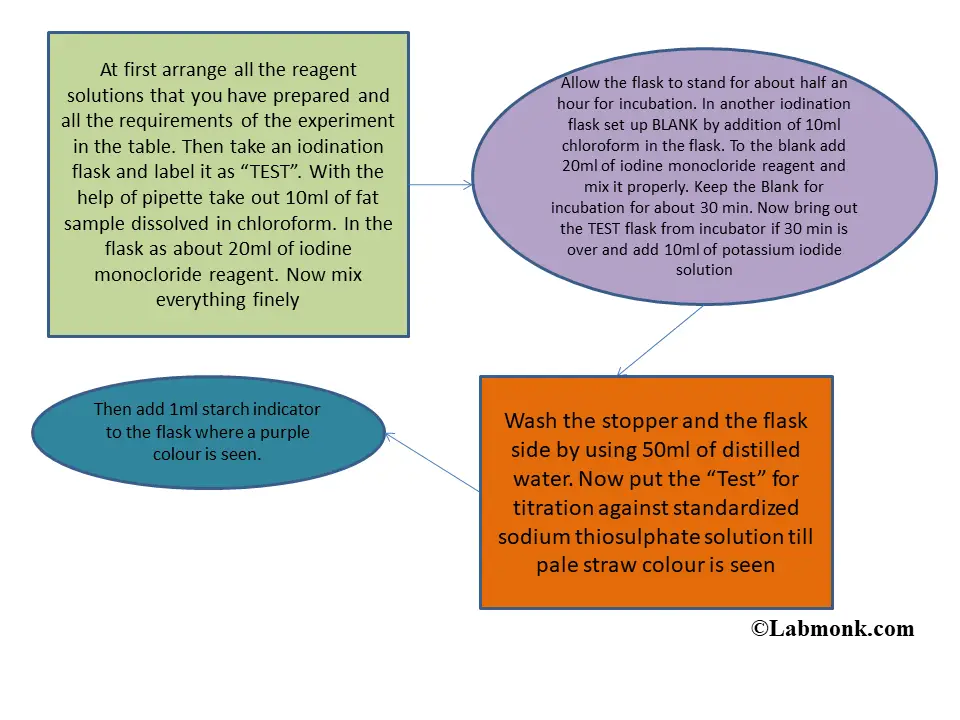
Determination Of Iodine Number Of Fat Labmonk

Lab Report 4 Iodine Value Docx Objective 1 To Evaluate The Quality Of Sunflower Oil Palm Oil And Corn Oil By Using Titration Techniques 2 To Course Hero






